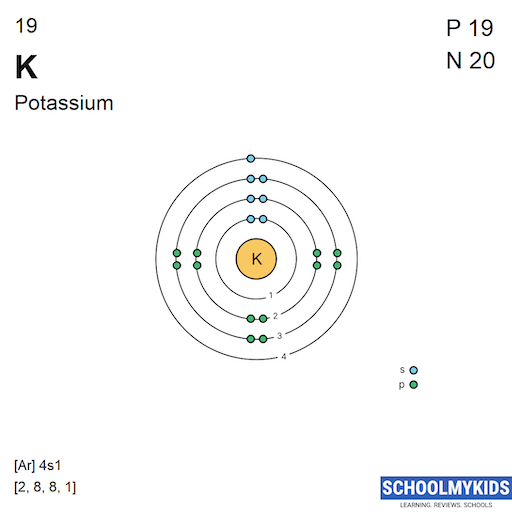
Bohr Model For Potassium
How to draw the Bohr-Rutherford Diagram for Potassium. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on.
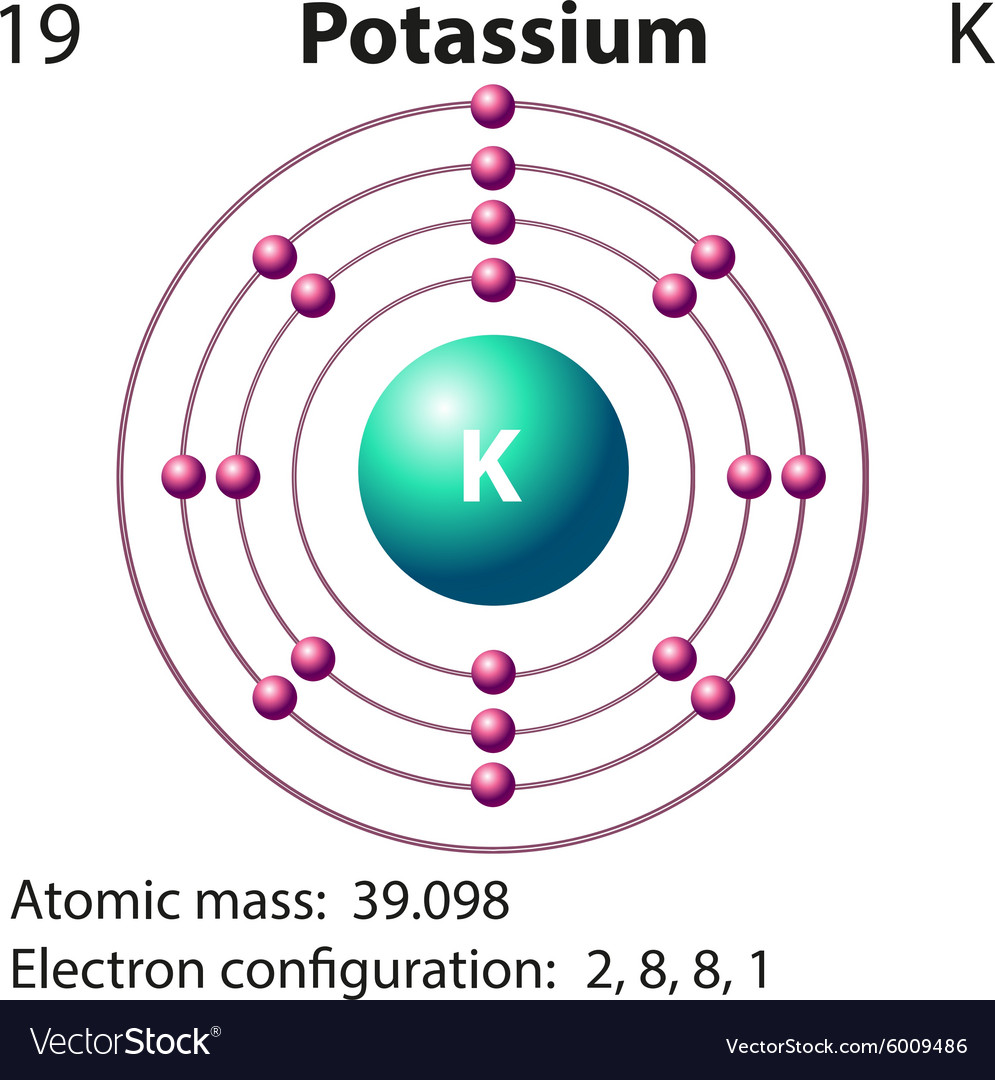
Diagram representation of the element potassium Vector Image
Steps Write protons, neutrons, and electrons of potassium atom Potassium has 19 protons, 20 neutrons, and 19 electrons. Learn how to find: Potassium protons neutrons electrons Draw nucleus of potassium atom The nucleus of a potassium atom contains 19 protons and 20 neutrons. So draw the nucleus of potassium atom as follows: Potassium nucleus

3d render of atom structure of potassium isolated over white background
Bohr's model calculated the following energies for an electron in the shell, n. . : E ( n) = − 1 n 2 ⋅ 13.6 eV. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. h ν = Δ E = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 eV.
Electron arrangements
The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to.

Bohr Model Potassium Atom Electron Structure Stock Vector (Royalty Free
1 λ = − ℜ( 1 n2 2 − 1 n2 1) Except for the negative sign, this is the same equation that Rydberg obtained experimentally. The negative sign in Equation 2.6.5 and Equation 2.6.6 indicates that energy is released as the electron moves from orbit n2 to orbit n1 because orbit n2 is at a higher energy than orbit n1.
Potassium Facts, Symbol, Discovery, Properties, Uses
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ).

What is the Bohr model for potassium? Quizlet
Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii. Britannica Quiz Matter and More Quiz How does Niels Bohr's atomic model work?

Potassium electron configuration. Illustration of the atomic structure
Here are a few highlights that you learned in the last section about Bohr's model:. Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4s subshell and calcium has an electron configuration of [Ar]4s 2. This gives calcium an outer-shell electron configuration corresponding.

Potassium Facts
2.8: Summary of Bohr's Contribution. Page ID. David M. Hanson, Erica Harvey, Robert Sweeney, Theresa Julia Zielinski. Chemical Education Digital Library (ChemEd DL) Bohr's proposal explained the hydrogen atom spectrum, the origin of the Rydberg formula, and the value of the Rydberg constant. Specifically it demonstrated that the integers in.
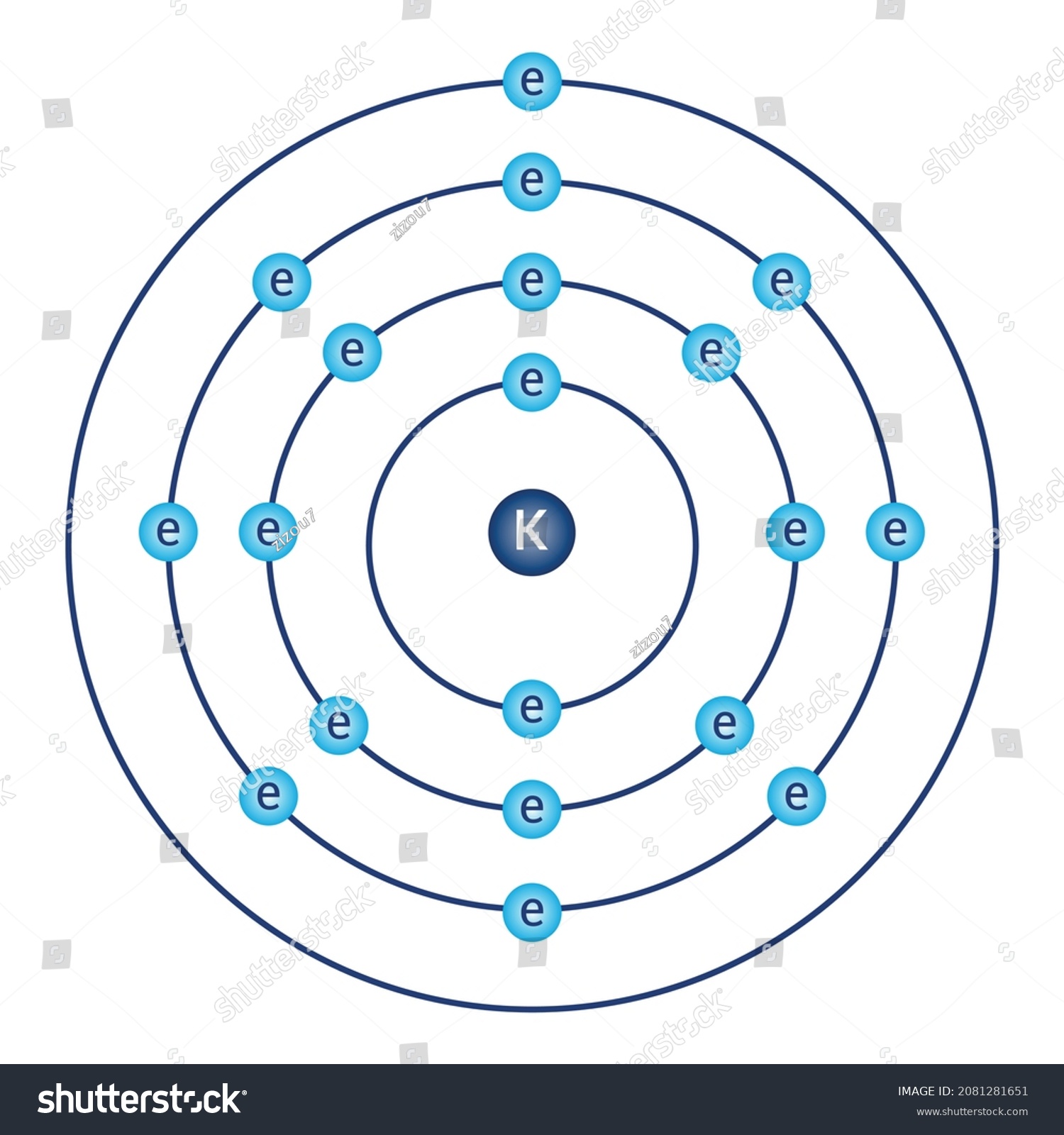
Bohr Model Diagram Potassium Atomic Physics Stock Vector (Royalty Free
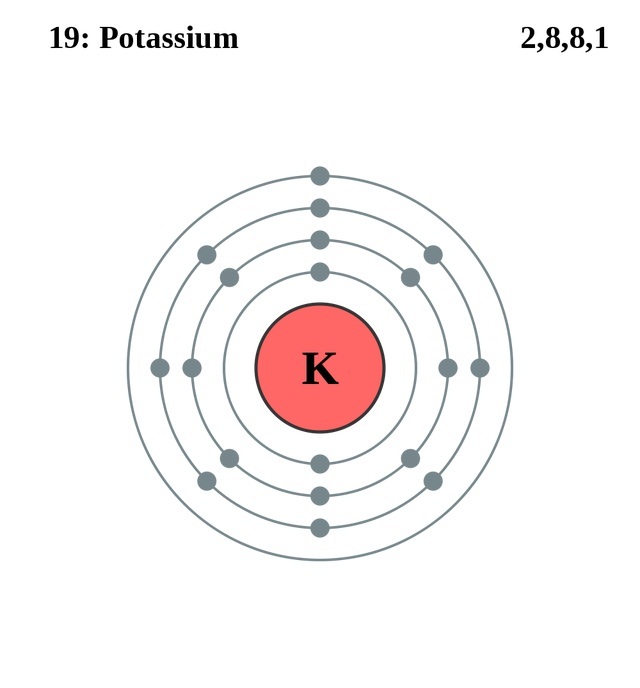
Potassium (K) atom electron configuration (Bohr model) Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund's principle, and Pauli's exclusion principle. Electron configuration of potassium through orbit Scientist Niels Bohr was the first to give an idea of the atom's orbit.
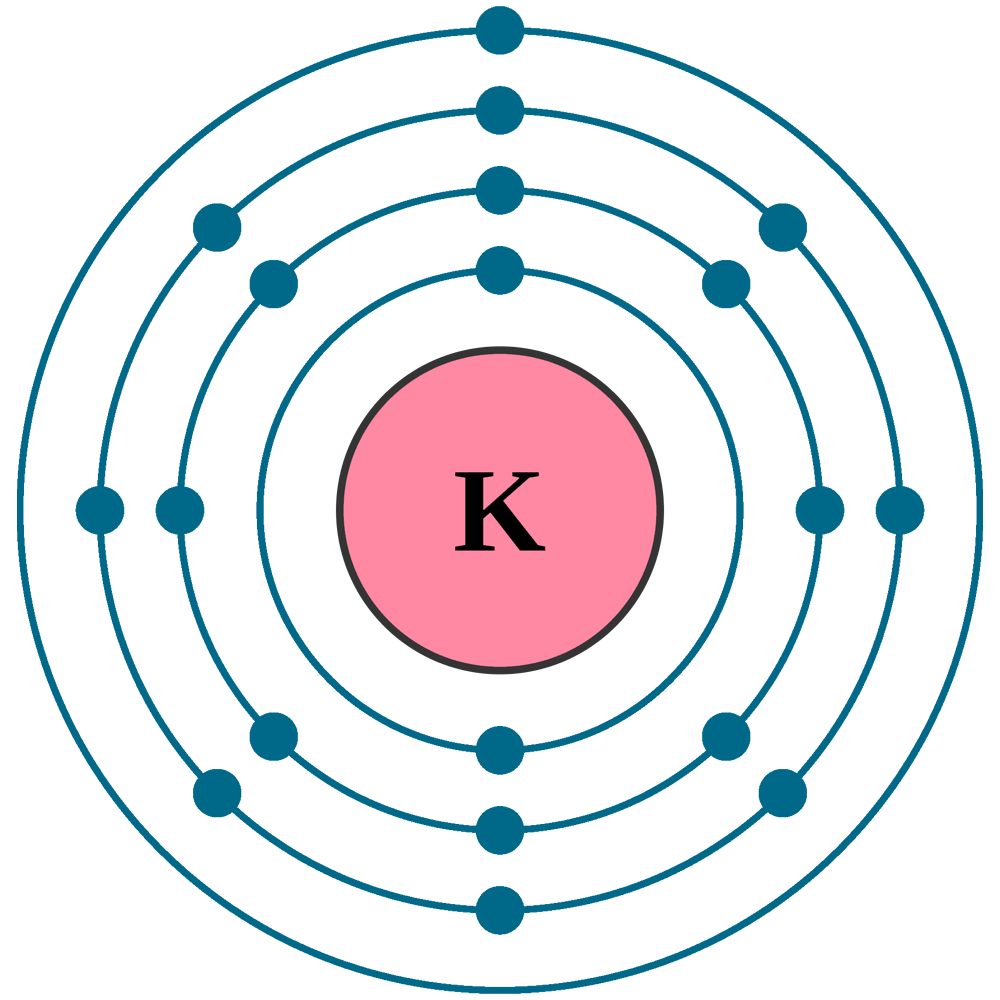
Potassium Electron Configuration Full Potassium Electron
What is the Bohr model of potassium? Chemistry Matter Basic Atomic Structure 1 Answer MathFact-orials.blogspot.com May 31, 2016 Please see the diagram below: Explanation: Answer link Please see the diagram below:
/potassiumatom-58b602485f9b5860464c55ea.jpg)
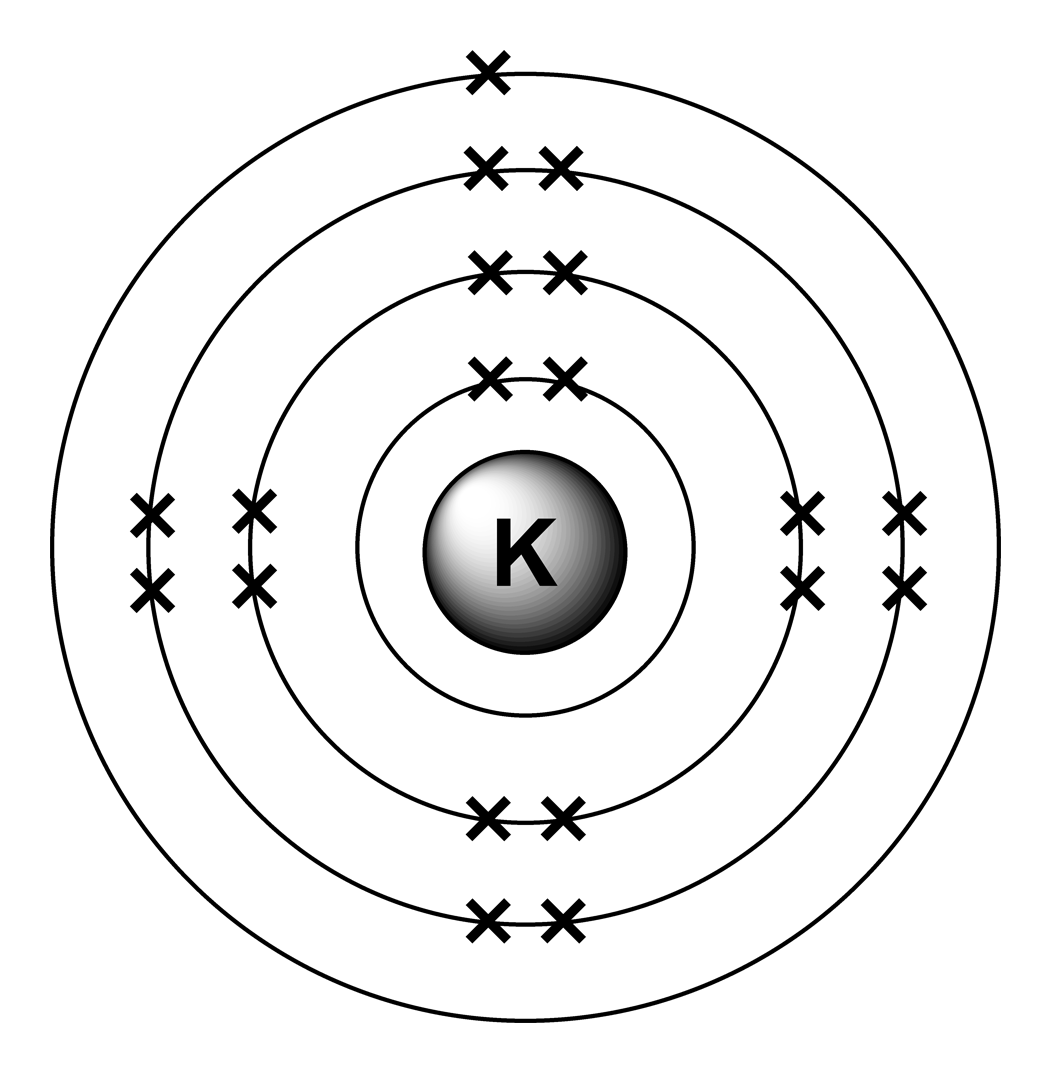
Atoms Diagrams Electron Configurations of Elements
The Bohr model represents the particle nature of electrons. So, it's easy to see that the atom above contains two electrons. As we'll discuss later in the article, atomic electrons exist at specific energy levels. The Bohr model represents these energy levels as rings.

Potassium Periodic Table Protons Neutrons And Electrons Awesome Home
An early model of the atom was developed in 1913 by the Danish scientist Niels Bohr (1885-1962). The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun.
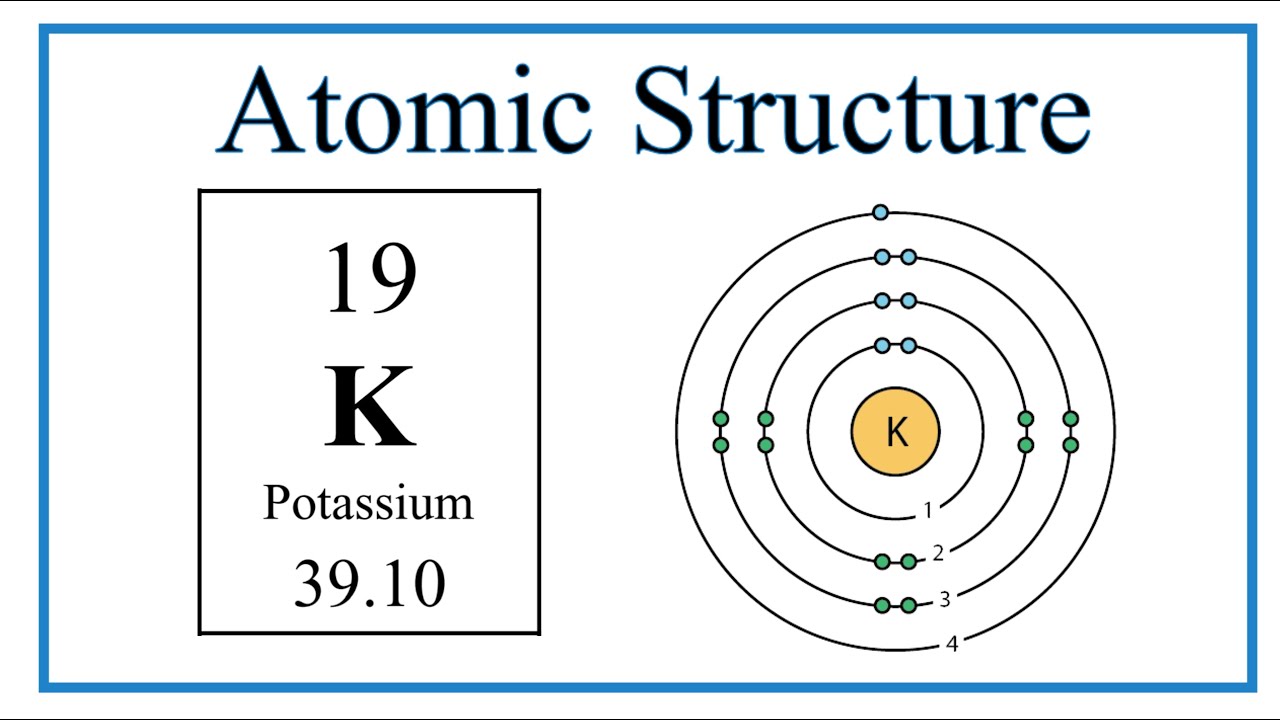
Atomic Structure (Bohr Model) for Potassium (K) YouTube
It is a very soft metal and can even be cut into pieces using a knife. It is silvery-white in appearance and is known to react vigorously with water. Potassium ions are known to play a vital role in our body such as nerve transmission and deficiency of potassium may result in abnormal heart rhythms.

diagrama de orbitales de potasio Brainly.lat
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
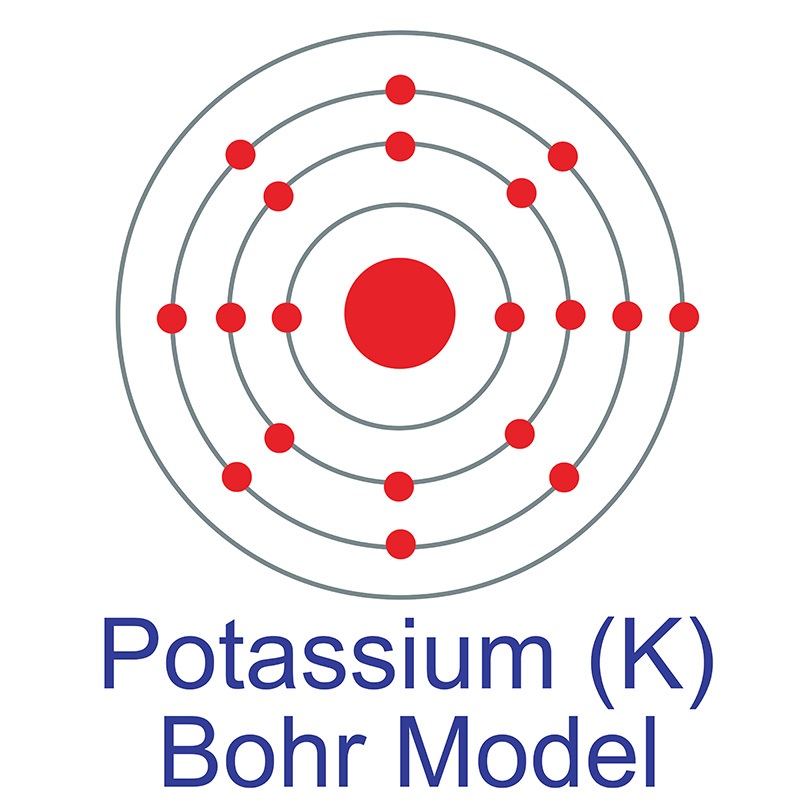
Optical parameters and dispersion behavior of potassium magnesium
The melting point of potassium is 63.5 °C and its boiling point is 759 °C. Potassium has many isotopes, but out of them 39 K has an abundance of around 93%. Density of potassium is less than the density of water. Hence it floats on water. Potassium is the 2nd lightest metal after lithium.